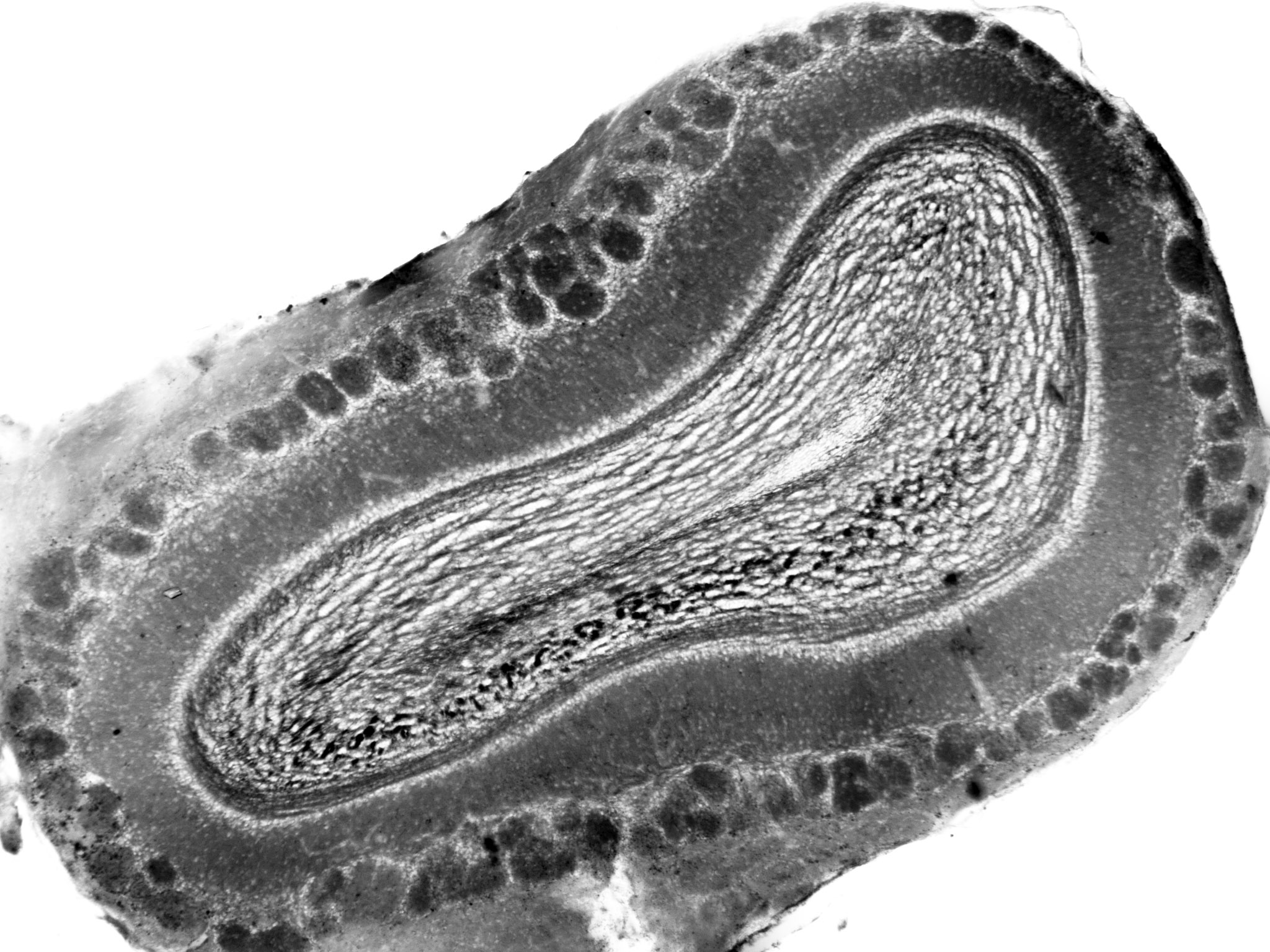
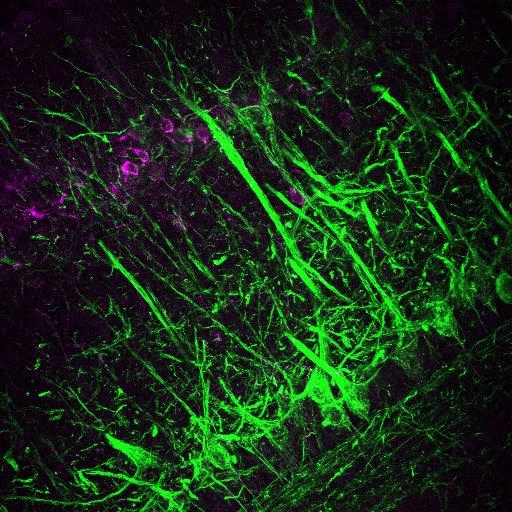
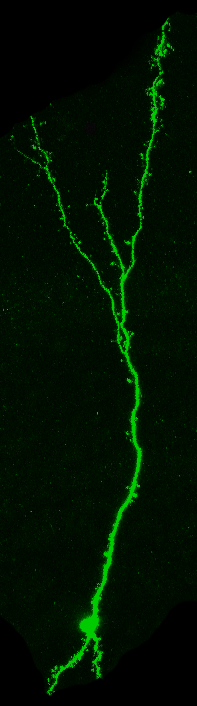
Our lab studies neuronal plasticity and its links to behaviour in the mouse olfactory system.
Faced with an ever-changing environment, animals must process sensory inputs to produce appropriate behavioural responses. Neuronal plasticity is fundamental to accomplish this but how adaptive control is achieved at the cellular and network level remains poorly defined.
Importantly, the way that circuits, such as those in the early olfactory system, respond and adapt to incoming signals needs to be optimized both for learning (e.g., to discriminate strawberry and banana) and also to assure homeostasis and behavioural stability (e.g., maintaining the ability to detect strawberry smell after temporary anosmia).
We are also fascinated by dopamine (it’s in the olfactory bulb too!), and by neurons capable of the most striking type of plasticity: the ability to undergo adult neurogenesis.

Neuronal Plasticity
The lab’s main aim is to further study the different types of neuronal plasticity, how they combine within individual cells at different stages of their lifetime, and how they impact on network processing as a whole.
To this end, we take an integrated approach to interrogate olfactory circuits at both the cellular and systems level, with a range of cutting-edge (i.e. optogenetics, chemogenetics and calcium imaging) and well-established technologies (i.e. electrophysiology and morphological techniques).
Collaborators:

Dopamine and adult neurogenesis
Although dopaminergic neurons are only a minority of brain cells, their impact on behaviour is substantial, and their impairment has been linked to numerous diseases. Recent studies have highlighted an extreme heterogeneity of morphology, physiology and connectivity among this rather small dopaminergic population, raising the question of whether these neurons have anything else in common besides dopamine itself.
To answer this question we are comparing features of the canonical midbrain dopaminergic cells with the relatively understudied group of dopaminergic neurons in the olfactory bulb, some members of which retain the striking ability to regenerate throughout life, aka the most extreme form of structural plasticity.
Collaborators:

Behaviour, learning, memory
We are developing open-source/low-cost olfactometers for precise odour delivery ,and fully automated behavioural testing setups to probe performance, learning and memory across multiple sensory modalities (mainly olfaction, but also vision and audition thanks to our collaborators). The goal is to link plasticity phenotypes at the cellular level with overall behavioural outputs.
Collaborators:
“When people ask me which disease we want to cure, I tell them that it is truly the worst of all: ignorance.”
Prof Jerry Simpson, circa 2010
Papers, preprints, datasets
Doyle C, Wang J, Galliano E, Guillaume C (2025) A low-cost and open-source olfactometer to precisely deliver single odours and odour mixtures.
bioRxiv 2025.09.11.675563; doi: https://doi.org/10.1101/2025.09.11.675563
Galliano E, Keck T (2025) Interactions between homeostatic plasticity and statistical learning: A role for inhibition. Current Opinion in Neurobiology, 93:103065, Systems Neuroscience issue on Statistical Learning
Gadiwalla S, Guillaume C, Huang L, White SJB, Basha N, Petersen PH, Galliano E (2025) Ex vivo functional characterization of mouse olfactory bulb projection neurons reveals a heterogenous continuum. eNeuro, 12 (3) ENEURO.0407-24.2025
→ Full dataset: Gadiwalla et al (2025). Research Data supporting “Ex Vivo Functional Characterization of Mouse Olfactory Bulb Projection Neurons Reveals a Heterogeneous Continuum”. Apollo – University of Cambridge Repository. https://doi.org/10.17863/CAM.114886
→ formerly known as the preprint: Ex vivo functional characterization of mouse olfactory bulb projection neurons reveals a heterogenous continuum. (2024) bioRxiv 2024.07.17.603915
Zhang Y, Pakulat LM, Takács S, Campbell L, Galliano E, Hrabovszky E, Colledge WH, Jones S (2025) Neuronal plasticity at puberty in mouse hypothalamic Kiss1 neurons that control fertility. Proc Natl Acad Sci U S A. 2025 Oct 28;122(43):e2512855122. doi: 10.1073/pnas.2512855122.
→ formerly known as the preprint: Zhang Y, Pakulat LM, Galliano E, Colledge WH, Jones S. Neuronal plasticity at puberty in hypothalamic neurons controlling fertility in female mice. bioRxiv 2024.10.06.616855. preprint
Doyle C, Guillaume C (2024) Reactivating an Engram: Context Matters. J Neurosci. 17;44(29):e0650242024. Journal club article.
Huang L, Hardyman F, Edwards M, Galliano E (2024) Deprivation-induced plasticity in the early central circuits of the rodent visual, auditory, and olfactory systems. eNeuro, ENEURO.0435-23.2023
→ Full dataset: Huang, L., Hardyman, F., Edwards, M., & Galliano, E. (2024). Research Data supporting “Deprivation-induced plasticity in the early central circuits of the rodent visual, auditory, and olfactory systems”. Apollo – University of Cambridge Repository. https://doi.org/10.17863/CAM.104411
→ formerly known as the preprint: Deprivation-induced plasticity in the early central circuits of the rodent visual, auditory, and olfactory systems: a systematic review and meta-analysis of the literature. (2023) bioRxiv 2023.09.04.556170
Lau MYH, Gadiwalla S, Jones S, Galliano E (2024) Different electrophysiological profiles of genetically labelled dopaminergic neurons in the mouse midbrain and olfactory bulb. . European Journal of Neuroscience, 1–20. “DOPAMINE: From Release and Modulation to Brain Diseases” special issue article
→ Full dataset: Lau, M. Y. H., Gadiwalla, S., Jones, S., & Galliano, E. (2024). Research Data supporting “Different electrophysiological profiles of genetically labelled dopaminergic neurons in the mouse midbrain and olfactory bulb”. Apollo – University of Cambridge Repository. https://doi.org/10.17863/CAM.102763
→ formerly known as the preprint: Characterization of Identified Dopaminergic Neurons in the Mouse Forebrain and Midbrain (2023) bioRxiv 2023.08.29.554772
→ highlighted by preLights here
Galliano E, Hahn C, Browne L, Rodriguez Villamayor P , Tufo C, Crespo A and Grubb MS. (2021) Brief sensory deprivation triggers cell type-specific structural and functional plasticity in olfactory bulb neurons. Journal of Neuroscience doi.org/10.1523/JNEUROSCI.1606-20.202. Full Dataset on KCL repository (doi.org/10.18742/RDM01-757).Cover of the March 10th 2021 issue.
Luppi AI, Newton CC, Folsom L, Galliano E, Romero-Garcia R. (2021) Ten simple rules for aspiring graduate students. PLOS Comp Bio; 17(8), e1009276. Editorial.
Galliano E, Franzoni E, Breton M, Chand AN, Byrne DJ, Murthy VN, Grubb MS. (2018) Embryonic and postnatal neurogenesis produce functionally distinct subclasses of dopaminergic neuron. eLife, 7:e32373 doi: 10.7554/eLife.323. Full Dataset On Dryad (doi.org/10.5061/dryad.b5hg8d6)
Galliano E, Schonewille M, Peter S, Rutteman M, Houtman S, Jaarsma D, Hoebeek FE, De Zeeuw CI (2018) Impact of NMDA Receptor Overexpression on Cerebellar Purkinje Cell Activity and Motor Learning. eNeuro, ENEURO.0270-17.2018.
Chand AN, Galliano E, Chesters RA, Grubb MS (2015) A distinct subtype of dopaminergic interneuron displays inverted structural plasticity at the axon initial segment. J Neuroscience, 35(4):1573-90.
D’Angelo E, Galliano E, De Zeeuw CI. (2015) The olivo-cerebellar system. Frontiers. Editorial.
Galliano E, De Zeeuw CI. (2014) Questioning the cerebellar doctrine. In Progress in Brain Research, Cerebellar Learning Volume, 210:59-77. Book chapter.
Galliano E, Gao Z, Schonewille M, Todorov B, Simons E, Pop A, D’Angelo E, van den Maagdenberg AM, Hoebeek F, De Zeeuw CI. (2013) Silencing the majority of cerebellar granule cells uncovers their essential role in motor learning and consolidation. Cell Reports, Apr 25;3(4):1239-51
Galliano E, Potters JW, Elgersma Y, Wisden W, Kushner SA, De Zeeuw CI, Hoebeek FE (2013) Synaptic transmission and plasticity at inputs to murine cerebellar Purkinje cells are largely dispensable for standard non-motor tasks. J Neuroscience, Jul 31;33(31):12599-618.
Galliano E, Baratella M, Sgritta M, Ruigrok TJH., Haasdijk ED, Hoebeek FE, D‘Angelo E, Jaarsma , De Zeeuw CI. (2013) Anatomical investigation of potential contacts between climbing fibers and cerebellar Golgi cells in the mouse. Frontiers in Neural Circuits, 7:59.
Badura A, Schonewille M, Voges K, Galliano E, Renier N, Gao Z, Witter L, Hoebeek FE, Chedotal A, De Zeeuw CI. (2013) Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron, May 22;78(4):700-13.
Galliano E, Mazzarello P, D’Angelo E. (2010) Discovery and rediscoveries of Golgi cells. J Physiol. 588(Pt 19):3639-55. Review.
Current and recent funding sources
Wellcome Trust, UKRI BBSRC and MRC, The Royal Society, Isaac Newton Trust, The Icelandic Research Fund (as co-PI)
Cambridge Trust (Li Huang), PDN-Wolfson College (Ailie McWhinnie), Harding Trust (Connor Doyle), Jesus College & Valluri-Rao (Harin Wijayathunga), Homerton College (Chloe Guillaume), Gates Cambridge (Tess Stanley)